Abstract
New diagnoses and relapses of primary immune thrombocytopenia (ITP) have been reported following SARS-CoV-2 infection and vaccination.
In a large monocenter cohort, we assessed: 1) incidence, risk factors and outcome of newly diagnosed ITP and ITP relapses after SARS-CoV-2 infection/vaccination; 2) incidence and risk factors for SARS-CoV-2 infection in the ITP cohort.
After IRB approval, 1142 ITP patients (pts) followed at our Hematology Center from 1982 to Feb 2022 were registered in an electronic database. Between Feb 2020 (pandemic start) and Feb 2022, 60 pts were newly diagnosed with ITP. Among the 1082 pts with ITP diagnosis prior to Feb 2020, 297 (27.4%) were deceased before the pandemic start and 403 (37.2%) were untraceable. Taken together, 442 ITP (60 newly and 382 previously diagnosed) pts were included in this study.
Information on SARS-CoV-2 infection/vaccination, platelet (PLT) count, and ITP therapy, was collected via phone call/e-mail or during routine medical visits. Laboratory data were verified through health electronic records. Risk factors for ITP relapse included sex, age, vaccine type, previous splenectomy, comorbidities (CCI), active disease (ongoing ITP therapy and/or PLT <100 x109/L), other immune diseases and ITP relapse to previous vaccine doses. Newly diagnosed ITP was considered infection-related (inf-ITP) or vaccine-related (vax-ITP) if the onset occurred <30 days from infection or vaccination, respectively. ITP relapse (rel-ITP) was defined as a drop in PLT count (<30 days from vaccination/infection) that required a rescue therapy OR a dose increase of an ongoing therapy OR a PLT count <30 x109/L with ≥20% decrease from baseline.
Eighteen (30%) out of 60 newly diagnosed pts had SARS-CoV-2-related ITP. Inf-ITP was diagnosed in 5 pts (8.3%). Compared to the 55 pts whose ITP was not related to Sars-CoV-2 infection, Inf-ITP pts were younger (median, 41.9 vs 70.2 yrs, p=0.02). ITP outcome was comparable in inf-ITP and other newly diagnosed pts, in terms of response to fist-line steroids (p=0.09), no. of therapeutic lines (p=0.9), and no. of pts still on therapy (p=0.9) at 6 months from diagnosis.
Vax-ITP was diagnosed in 13 (21.7%) pts after the first (no.4, 30.8%), second (no.4, 30.8%) or third vaccine dose (no.5, 38.4%). Vaccines were: Comirnaty (53.8%), Vaxzervria (23.1%) or Spikevax (23.1%). Compared to the 47 pts whose ITP was not related to Sars-CoV-2 vaccine, Vax-ITP pts were older (median, 74 vs 55 yrs, p=0.04). ITP treatment duration was longer in Vax-ITP pts (median, 150 days vs 54 days in no Vax-ITP pts, p=0.04); 53.8% of vax-ITP pts were still on therapy 6 months after diagnosis (vs 23.4%, p=0.03).
Between Feb 2020 and Feb 2022, 69/382 (18.1%) had a relapse of ITP. In 36/69 pts (52.2%), relapse was related to SARS-CoV-2 infection (1 case) or vaccination (35 cases out of 360 pts who received ≥1 vaccine dose, 9.7%). Ten pts experienced multiple relapses (46 total cases). Post-vaccine relapse occurred in 3.3%, 4.7%, and 5.4% of these 35 pts after the 1st, 2nd, and booster dose, respectively (p=0.65).
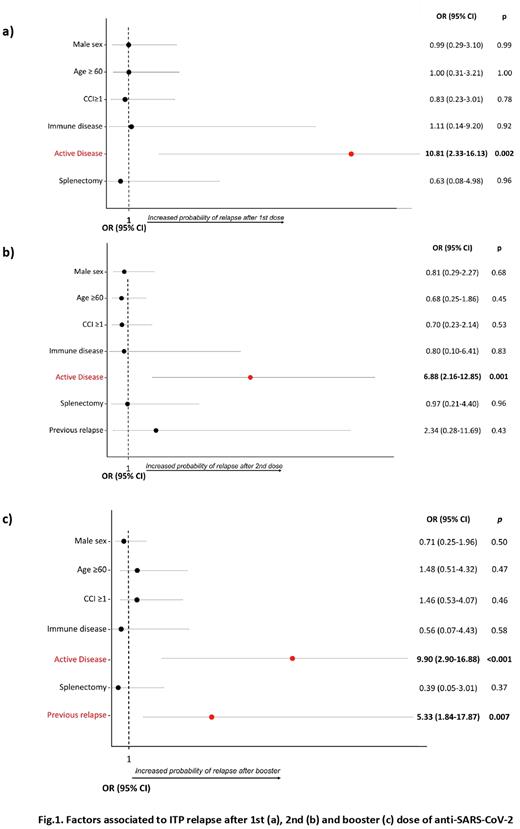
In multivariate analysis (MVA), active disease at the time of the 1st and the 2nd vaccine dose was associated with Rel-ITP (Odds ratio, OR 10.81, p=0.002; OR 6.88, p=0.001, respectively). After booster, active disease (OR 9.90, p<0.001) and previous Rel-ITP (OR 5.33, p=0.007) was associated with relapse (Fig.1). No major bleeding occurred in newly diagnosed and relapsed pts.
Overall, 89/442 (20.1%) ITP pts had a SARS-CoV-2 infection (g≥3 in 10.1%). In MVA with death as competing risk, ≥2 vaccine doses (SHR 0.18; p<0.001) was associated with a lower risk of SARS-CoV-2 infection.
SARS-CoV-2 is a risk factor for ITP onset, with 30% of new diagnoses being related to infection/vaccination. In older pts, the risk of vax-ITP is higher, and ITP requires more prolonged therapy, suggesting closer hematological monitoring. Also, >50% of total ITP relapses were related to SARS-CoV-2, mainly occurring after vaccination and regardless of vaccine dose. Thus, strict hematological monitoring may be recommended after all doses. In pts with active disease at vaccination and/or previous ITP relapse after vaccine, the completion of the vaccine program and subsequent laboratory follow-up should be personalized. Notably, vaccination confirmed to be the most protective factor against SARS-CoV-2 infection also in ITP pts.
Disclosures
Cavo:Janssen: Honoraria, Speakers Bureau; AbbVie, Amgen, Bristol Myers Squibb/Celgene, Pfizer, GlaxoSmithKline, Sanofi, Roche, Takeda: Consultancy, Honoraria. Palandri:Sierra Oncology: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; AOP: Consultancy, Honoraria; Sobi: Consultancy, Honoraria; CTI: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Kartos/Telios: Consultancy, Honoraria; Amgen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal